Research
Overview
Energy storage materials and devices are among the most critical infrastructures needed to power future smart and resilient cities, where high energy, high power, high mobility, and reliable safety are all essential. Our research focuses on various energy storage chemistries tailored for diverse application scenarios, including—but not limited to—metal batteries (Li, Na, etc.), all-solid-state batteries, multivalent-ion batteries, and large-scale grid storage.
Our work integrates multiple engineering disciplines and advances in the following directions:
New materials and advanced manufacturing for energy storage and mobility – Designing and integrating next-generation functional materials with scalable fabrication methods to enable high-performance, safe, and manufacturable batteries.
Multidimensional quantitative characterization – Developing and applying operando, spatially resolved, and cross-scale diagnostic techniques for failure analysis, mechanistic understanding, and method standardization.
Data-driven materials and device design – Utilizing quantitative datasets, modeling, and machine learning to accelerate the discovery, optimization, and validation of functional energy materials and devices.
Critical materials and circular supply chains – Advancing copper metallurgy, critical material recovery, recycling technologies, and domestic manufacturing strategies to strengthen the energy storage and electrification supply chain.
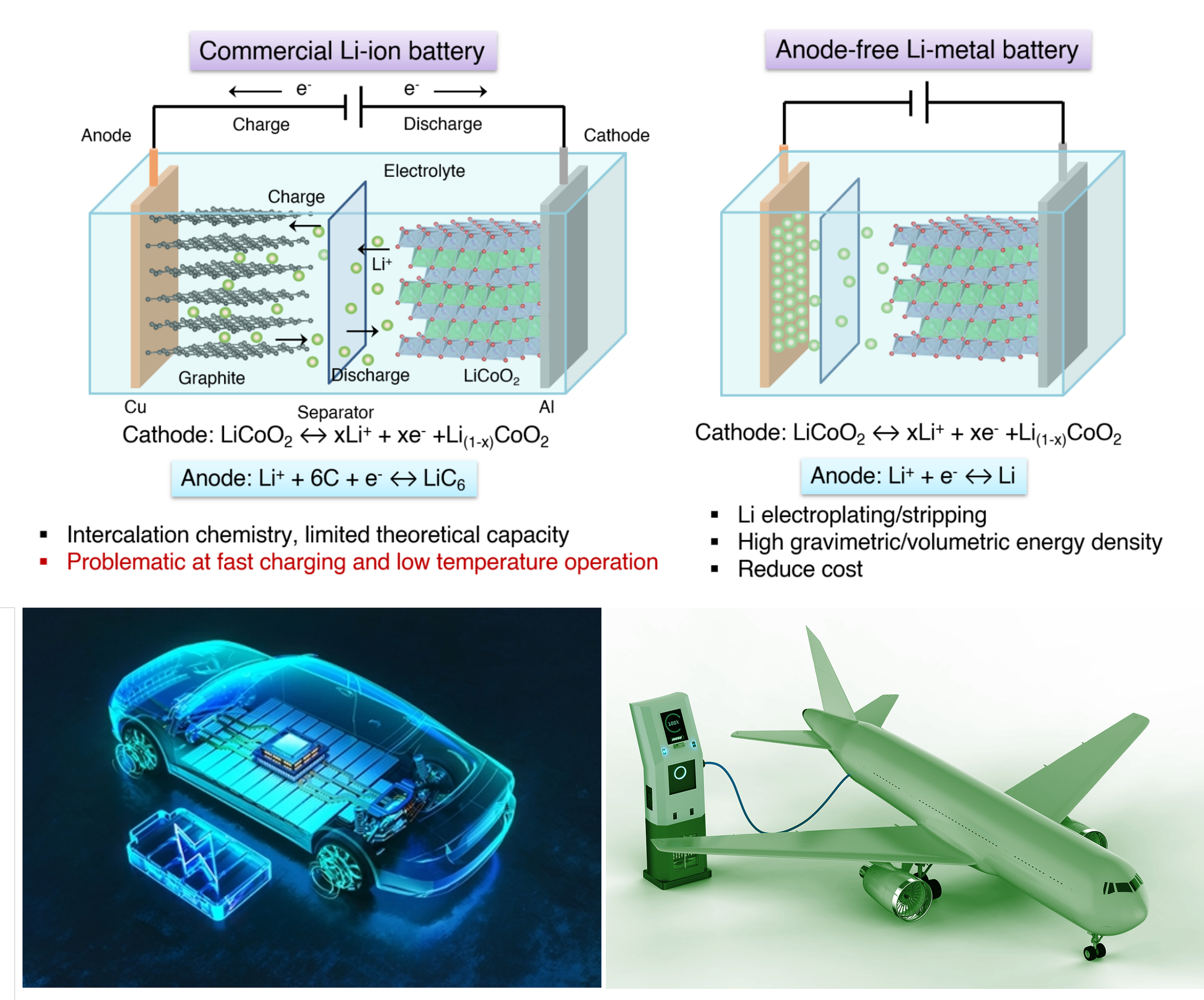
Anode-Free Lithium Metal Batteries
In commercial lithium-ion batteries, the lithium-containing cathode provides the capacity, while the graphite anode only serves as a safe host for lithium ions. Eliminating the graphite anode, known as the anode-free lithium metal battery (AFLMB) — opens a pathway to achieving cell-level energy densities of 500 Wh/kg and beyond. Recent innovations in lithium metal batteries are now being recognized as major milestones in both academia and industry, advancing the long-standing goal of practical lithium metal batteries. These breakthroughs will not only double the driving range of electric vehicles, but also enable entirely new technologies that are currently limited by power constraints, such as electrified aircrafts and other high-energy-demand systems. The essence of AFLMB lies in enabling dendrite-free and highly efficient electrochemical cycling of lithium metal, which requires synergistic advancements in electrolyte design (both liquid and solid), deeper fundamental understanding of lithium electrochemical behavior, and system-level integration.
Reference: “Key issues hindering a practical lithium-metal battery”, Trends in Chemistry, 2019, 1, 152-158
Advanced Manufacturing
We focus on establishing an integrated manufacturing pipeline for high-performance batteries, bridging fundamental materials chemistry with system-level engineering. We begin by designing and optimizing electrode and electrolyte materials—such as solid-state interfaces and high-capacity cathodes—to address inherent challenges in ion transport, stability, and safety. These materials are then translated into manufacturable formats through process engineering and scalable fabrication methods, including roll-to-roll coating, slurry casting, calendaring, and cell assembly. Finally, we integrate these cells into battery modules and full pack systems, where thermal management and structural design are critical for ensuring long-term reliability and safety under real-world operating conditions. By coupling materials innovation with scalable manufacturing and pack-level optimization, our goal is to enable next-generation energy storage technologies that accelerate electrification for transportation and resilient infrastructure.
Pressure-Tailored Materials Growth
Our research pioneers the use of pressure as an active design parameter to precisely control materials growth, microstructure evolution, and electrochemical performance. Building on recent advances in pressure-regulated lithium deposition, we develop pressure-tailored growth strategies to manipulate nucleation pathways, promote lateral densification, and suppress defects and void formation at the nanoscale. By integrating operando characterization, cryo-FIB/SEM–TEM imaging, and multiscale simulations, we revealed that optimized uniaxial pressure can direct columnar grain formation, maintain interface integrity, and drastically reduce dead material accumulation, leading to 99%+ reversibility and high-rate stability. This pressure-based methodology provides a universal knob to engineer crystalline orientation, interface cohesion, and energy transport in diverse functional materials beyond batteries—including metallic films, 2D layered materials, and composite scaffolds. Ultimately, our pressure-tailored growth framework opens a new paradigm for manufacturable, high-performance materials, enabling scalable synthesis routes for next-generation energy devices and sustainable fabrication platforms.
Reference: “Pressure-tailored lithium deposition and dissolution in lithium metal batteries”, Nature Energy, 2021, 6, 987–994
Critical Minerals
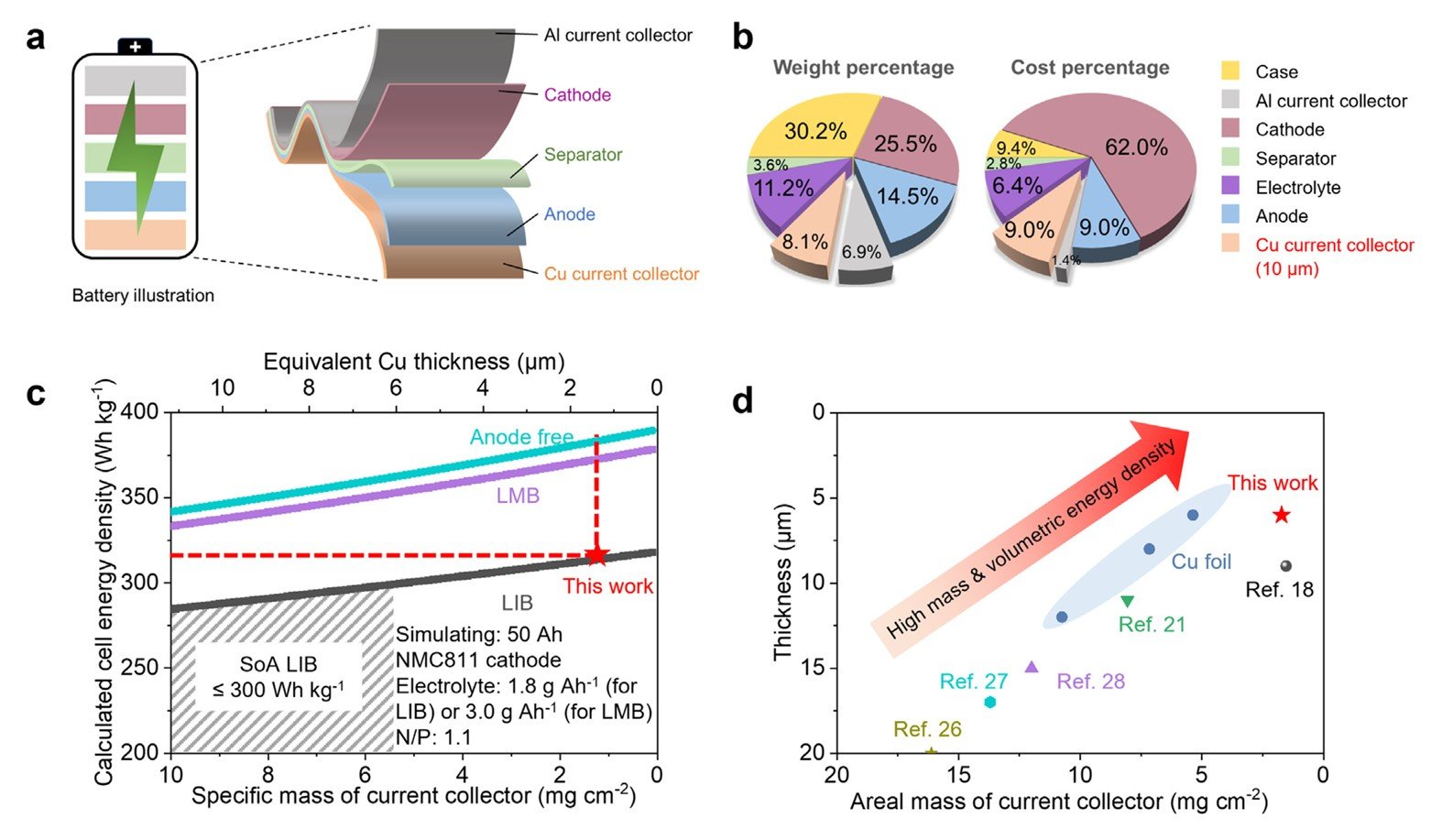
We are broadly interested in critical minerals and low-cost, sustainable battery materials that can support large-scale electrification without stressing fragile supply chains. We focus on copper and other key metals used in current collectors and battery components, asking how to use less, waste less, and source smarter. By integrating ultralight current-collector architectures, scalable electroless coating, and advanced copper metallurgy, we reduce metal intensity and cost at the cell level while maintaining or improving energy density and safety. In parallel, we study recycling and circular pathways—recovering copper and other critical elements from manufacturing scrap and end-of-life batteries, and reintegrating them into high-performance components. Together, these efforts aim to couple fundamental materials design with resource efficiency, enabling resilient, domestically robust supply chains for next-generation energy storage.
Reference: “Fabricating ultralight and ultrathin copper current collectors for high-energy batteries”, eScience, 2024, 4, 100271.
Multiscale Characterization and Diagnostics
Titration Gas Chromatography
Inactive or “dead” Li formation is the immediate cause of capacity loss and safety hazards of high-energy lithium metal batteries; it consists of both (electro)chemically formed Li+ compounds in the solid electrolyte interphase (SEI) and electrically isolated unreacted metallic Li. However, quantitatively distinguishing between Li+ in SEI components and the unreacted metallic Li has not been possible due to the lack of effective diagnosis tools. We established a new analytical method, TGC, and accurately quantified the contribution from unreacted metallic Li to the total amount of inactive Li. We identify the unreacted metallic Li, rather than the (electro)chemically formed Li+ in SEI, as the dominant source of inactive Li and capacity loss.
Reference: “Quantifying inactive lithium in lithium metal batteries”, Nature, 2019, 572, 511–515
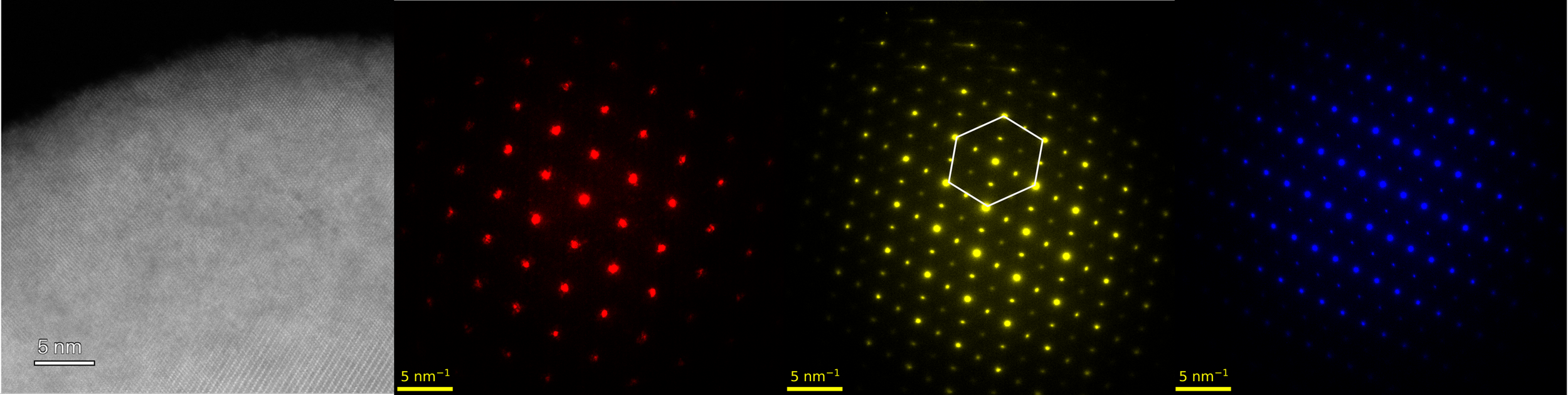
Cryogenic Transmission Electron Microscopy
Cryo-TEM is a powerful tool to probe the nanostructures of electrochemcially active materials, such as lithium metal and its SEI. It is impossible to acquire high-resolution images of these materials at room temperature due to their extremely sensitive nature to electron beam. The cryogenic protection minimizes the beam damage to the brittle materials while preserve its intrinsic properties.
Reference: “Quantifying inactive lithium in lithium metal batteries”, Nature, 2019, 572, 511–515
Cryo-EM facility at MSU: Center for Advanced Microscopy
Cryogenic Focused Ion Beam
FIB-SEM images provide the cross-section morphology information of materials. Cryogenic protection is critical because the electrochemically active materials are not only sensitive to electron beam, but also is apt to react with the FIB incident ion beam at room temperature. Taking a series of cross-sectional FIB-SEM images, a 3D structure can be reconstructed, enabling 3D visualization and quantitative structural analysis.
Reference: “Pressure-tailored lithium deposition and dissolution in lithium metal batteries”, 2020, under review
Cryo-FIB facility at MSU: Composite Materials and Structures Center
Materials by Design
Surface Modification
Graphene oxide/polydopamine-coated Si nanocomposite (GO/PDA-Si) was synthesized by a novel facile solution-based chemical method at room temperature. The surface property of Si nano particles (NPs) was modified by introducing secondary amine groups from PDA, which form amide groups with carboxyl groups and hydrogen bonds with hydroxyl/carboxyl groups on GO. These chemical interactions firmly anchor Si NPs to GO so that aggregation of Si NPs can be mostly prevented.
Reference: “Improving the electrochemical performance of Si nanoparticle anode material by synergistic strategies of polydopamine and graphene oxide coating”, Journal of Physical Chemistry C, 2015, 119 (4), 1720–1728
Bulk Doping
Oxygen-redox reactions in lithium-rich layered oxide cathode materials enable ultra high capacity, but causes voltage decay due to the unwanted oxygen gas formation. We firstly synthesized Li[Li0.2Ni0.2Mn0.6]O2 cathode materials by the modified co-precipitation method. Guided by the ab initio calculations of oxygen vacancy formation energy, we then selectively chose the Co and Mo co-doping into the Li[Li0.2Ni0.2Mn0.6]O2 materials with the aims to facilitate oxygen activity while mitigate the voltage decay. The co-doping design enhances both capacity and cycling stability.
Reference: “Modified co-precipitation synthesis of meso-structure controlled Li-rich layered oxides for minimizing voltage degradation”, ACS Applied Energy Materials, 2018, 1, 3369
“Mitigating oxygen release in anionic-redox-active cathode materials by cationic substitution through rational design”, Journal of Materials Chemistry A, 2018, 6, 24651